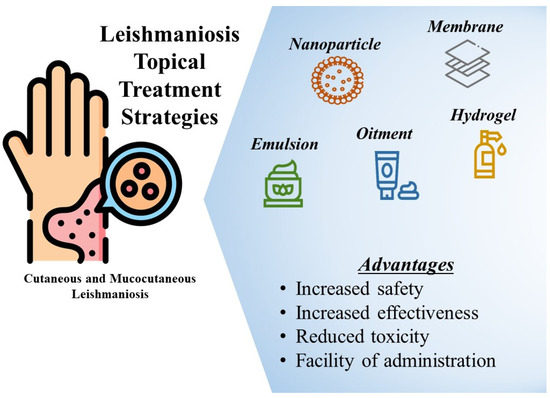
LeishmaniasisLeishmaniasis Overview Leishmaniasis is a parasitic disease transmitted through the bite of infected sand flies. It is associated with poverty and occurs in areas with poor housing, migration, and environmental changes. The disease has three main forms: * Visceral leishmaniasis (kala-azar): A severe form characterized by irregular fever, weight loss, enlarged spleen and liver, and anemia. * Cutaneous leishmaniasis: The most common form, causing skin ulcers. * Mucocutaneous leishmaniasis: Affects the mouth, nose, and throat. Visceral leishmaniasis is primarily found in Brazil, East Africa, and India. Early detection and treatment are crucial for controlling the disease. Diagnosis Symptoms of leishmaniasis are non-specific, so diagnosis is confirmed through laboratory tests: * Serological tests (DAT, rK39): Detect antibodies against Leishmania. * Rapid Diagnostic Test (RDT): Detects antibodies using a simple test that can be used in remote areas. However, as antibodies can persist after healing, serological tests cannot be used to determine healing or relapse. The tests may also show false positives in healthy individuals living in endemic areas. Monitoring Treatment After treatment, monitoring healing requires a non-invasive assay with good sensitivity, specificity, and reproducibility.
Overview
Leishmaniasis is caused by protozoan parasites transmitted by the bite of infected female phlebotomine sand flies. The disease is poverty-related and has been associated with poor housing, migration and movement of nonimmune people to areas with established endemic or enzootic transmission cycles, environmental and climatic changes, protein-energy malnutrition, immunodeficiency, and lack of resources.
There are three main forms: visceral leishmaniasis, also known as kala-azar, which is the severe form because it is fatal if not treated promptly, and cutaneous leishmaniasis, which is the most common form and causes skin ulcers and mucocutaneous ulcers that affect the mouth, nose and throat.
Visceral leishmaniasis is characterized by irregular attacks of fever, weight loss, enlargement of the spleen and liver, and anemia. Most cases occur in Brazil, East Africa and India. It is estimated that 50,000 to 90,000 new cases of visceral leishmaniasis occur worldwide each year.
Early detection and appropriate treatment are key strategies for the control of visceral leishmaniasis. Signs and symptoms of the infection are nonspecific; The diagnosis is therefore confirmed by combining clinical symptoms with Leishmania-specific laboratory tests. The diagnostic policy for health care in endemic areas depends on the level of the health care system. Two serological tests—the direct agglutination test and the rK39 antigen-based immunochromatographic tests—have been developed for field use in most endemic areas (3). A rapid diagnostic test (RDT) detects antibodies and is a simple test that can be used at both peripheral and central levels. A scientific review of published studies estimates that the sensitivity of RDTs varies depending on eco-epidemiological regions, particularly low sensitivity in East Africa (4,5). Given the persistence of antibodies for long periods after healing, all serological tests have their limitations in that they cannot be used as a test for healing or to diagnose relapse. Also, a significant proportion of healthy people living in endemic areas without a history of visceral leishmaniasis test positive for antileishmanial antibodies due to asymptomatic infections. Therefore, antibody-based tests should always be used in conjunction with a standardized clinical case definition for the diagnosis of visceral leishmaniasis.
Similarly, monitoring treatment after healing requires a non-invasive in vitro assay for healing, which should have good sensitivity and specificity and be reproducible and feasible to use.